
Claire Robinson asks whether claims that GMO virus-resistant cassava could “feed millions” hold up
Plant genetic engineer Devang Mehta has quit GMO research, claiming that public “backlash and criticism” of GM technology is to blame for the fact that universities across Europe are shutting down their GMO research programmes. This retreat, according to Mehta, has resulted in the world’s poor being deprived of two GMOs that could “help feed millions”. These are golden rice and the virus-resistant cassava – the latter forming the subject of his own research.
In Part 1 of this series, I investigated GMO golden rice and found that the real reason it’s not in farmers’ fields has nothing to do with public opposition to GM and everything to do with the development problems that have plagued the crop.
But what of cassava? A GM virus-resistant cassava has for many years been a “holy grail” of the GMO lobby: long sought, but never found. As with golden rice, despite large dollops of hype being regularly served up by the media, the GM virus-resistant cassava exists only in the fevered imaginations of career plant genetic engineers and their funders.
Virus resistance has proved difficult to genetically engineer into cassava in a stable way, with one project after another ending in failure. Research has been led by the Donald Danforth Plant Science Center in the US, which was launched with with a $50 million gift from Monsanto.
In 2005 the Danforth Center boasted that it could “feed the continent” of Africa with cassava resistant to the cassava mosaic virus (CMV) – if it were not for the “confusion and fear” generated by anti-GMO campaigners.[1] By 2006 it was forced to admit that its experimental GM cassava had lost resistance to the virus.[2]
Fast forward to 2015 and once again researchers from the Danforth Center, this time in collaboration with scientists from Uganda, were having to admit that another GM virus-resistant cassava had lost resistance to CMV. According to their published paper, this was due to the effects of the particular type of tissue culture process that has to be used with all commonly used genetic engineering methods in cassava.[3]
Tissue culture the culprit
Somewhat ironically, the authors note that the type of cassava that they were trying to engineer, TME 204, which belongs to a class called CMD2‐type cultivars, was naturally resistant to CMV but lost this resistance during field cultivation: “The data… show that the CMD2 resistance mechanism was negated in plants of transgenic lines of TME 204 cultivated under field conditions.” The cause of this loss of resistance was passing the plants through a process called tissue culture, which is a necessary phase of the GM plant development method.[3]
CMD2 cultivars are susceptible to another virus that is affecting the crop in Africa, cassava brown streak disease (CBSD). Thus the Danforth researchers had genetically engineered the trial cassava with an RNAi (RNA interference/gene silencing) construct to try to confer resistance to CBSD. The idea here is that the RNAi molecules engineered into the cassava target and silence the function of crucial genes of the virus that cause CBSD, thus stopping it in its tracks when it infects the plant.
Could the loss of CMV resistance have been due to genetic disruption stemming from the RNAi molecules engineered into the plants? The authors rejected this hypothesis when they found that passing the plants through tissue culture, regardless of whether or not they contained RNAi transgene sequences, was sufficient to cause the resulting plants to become susceptible to CMV infection.[3]
Danforth link with ETH Zurich
That’s all very well, you might say, but what does this Danforth work have to do with Mehta?
It turns out that the Danforth Center is linked to Mehta’s university, ETH Zurich, through an initiative called the Global Cassava Partnership for the 21st Century (GCP21).
Mehta did his cassava work at the Plant Biotechnology Group at ETH Zurich under the group head, Wilhelm Gruissem. Gruissem sat on the international committee of the second scientific conference of the GCP21.
The GCP21 claims that it consists of 45 member institutions working on the research and development of cassava, but the part of its website listing its partners contains no information. However, GCP21 is chaired by Dr Claude Fauquet of the Danforth Plant Science Center. The GCP21’s second scientific conference was sponsored by the Danforth Center, as well as the Bill & Melinda Gates Foundation, “USAID from the American People” [sic.], and the GMO firms Monsanto, Syngenta, and Cibus.
Monsanto’s presence on the GCP21 event is significant. Mehta wrote of his ETH Zurich research group, “We are not funded by Monsanto, and our GMOs are largely patent-free”.
But this statement is either naïve or deliberately misleading. If the ETH Zurich group’s publicly funded research were to come up with a commercially viable GMO, Monsanto or a similar company would, in all likelihood, step in to file patents and organize licensing agreements. GMO firms have a long history of appropriating the outcomes of publicly funded research for their own private profits. And universities themselves now operate as businesses, with whole departments dedicated to establishing Intellectual Property Rights on their researchers’ discoveries.[4]
Did Gruissem drive UC Berkeley into Big Biotech’s lap?
Mehta’s mentor Gruissem is a ripe candidate for maximizing the commercial potential of any GMO product.
In the late 1990s, before Gruissem took up his post at ETH Zurich, he was at the University of California at Berkeley (UC Berkeley). According to Ignacio Chapela, a professor of environmental science, policy and management at UC Berkeley, Gruissem was “responsible for driving my university into the lap of Novartis (now Syngenta), in a first experiment in what are now known as PPPs or private-public partnerships”.
Prof Chapela was an outspoken opponent of the deal. He said, “The principled opposition to this idea by many faculty on campus, and the outrageous terms under which a profit-driven Swiss company pretended to capture public resources in the US, made the proposal slow down slightly, though not completely stop.”
In November 1998, UC Berkeley signed a five-year $25 million research agreement with Novartis. The arrangement would give UC Berkeley’s Department of Plant and Microbial Biology access to research funds as well as to Novartis’ genetic sequencing databases. In return, Novartis held the first rights to patent discoveries made over the five-year period.[5]
In the midst of the delay caused by the opposition to the deal, by Prof Chapela’s account, Gruissem left the US for his current chair at the ETH Zurich, where his research is focused on cassava as well as rice and wheat.
Gruissem’s ETH Zurich profile states, “Cassava, the staple crop of more than 800 million people worldwide and also commercially important for its high-quality starch, is affected by severe virus diseases in Africa and India that are now threatening Asian countries as well. Moreover, cassava yields in many countries are often far below their agronomic potential.”
The profile adds, “We recognize the importance of applying our biotechnology skills to provide solutions in major staple crops.”
GM virus resistance fails
How has Gruissem’s department’s research progressed in offering GMO “solutions” to the problems with cassava?
A first clue lay in a Twitter exchange on 20 September 2017 between GMO promoter Prof Kevin Folta and Devang Mehta. Folta tweeted, “Ugandan biotech cassava field trials show another success in virus resistance, protecting a crop that feeds >800 M.”
But Mehta replied, “Wouldn’t advert this a lot. The transgenic broke existing nat. resistance to diff. more widespread virus. My lab’s working on this tho.”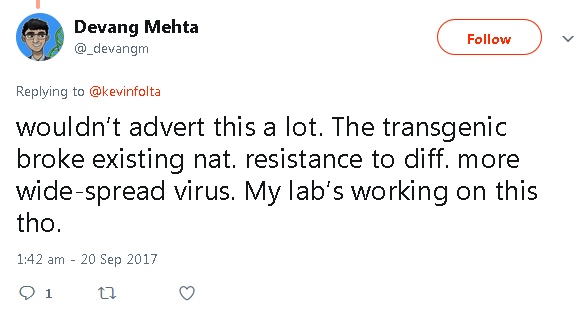
Mehta appears to be saying that the genetic engineering process destroyed the plants’ natural resistance to a different, more widespread virus.
Once again, attempts to genetically modify cassava to overcome viral disease had failed.
It was following this failure that Mehta penned his article announcing that he was leaving GMO research. Notably, he did not admit to the inherent problems of the technology, choosing instead to blame public “backlash and criticism” of GM.
Central Dogma proves hollow
Ignacio Chapela believes that Mehta’s exit signals a trend. He points to the “Central Dogma” of molecular biology: "DNA makes RNA and RNA makes protein". This Dogma was always over-simplified and completely inadequate to explain the complexities of gene function. Nonetheless, Prof Chapela believes that it still forms the shaky foundation on which the plant genetic engineering venture bases itself and that it is still held up to the public as a sacred and unquestionable truth.
Prof Chapela said, “While the level of commitment (institutional, conceptual, financial, political) to a pseudo-biology based on the ideology of Central Dogma has reached a level of collective hysteria, biologists who are more serious about their subject have been slowly and quietly moving away from the field, for good reason: Central Dogma has proven hollow at best, and woefully misleading and damaging in its more pernicious versions.
“The tools of molecular biology and the hyper-industrialization of tasks such as sequencing and DNA amplification have yielded useful information. But between this ‘revolution’ and the wild promises of the aging gene-jockeys and their disciples, any self-respecting biologist knows there is an unbreachable gap. It is not ‘backlash and criticism’ that has closed those labs in Europe and elsewhere – I wish the public resistance to their very real political, social and ecological consequences had been enough to even slow them down. Most of their failures are driven by their own obvious conceptual bankruptcy.”
GM cassava research: Dangerous as well as failed
As this article was being prepared for publication, Mehta, Gruissem and colleagues published a paper on a pre-journal publication website about their research in which they attempted to engineer resistance to disease-causing geminiviruses into cassava using the CRISPR-Cas9 “genome-editing” system. Their aim was to engineer the CRISPR-Cas tool into the cassava together with a guide RNA targeting two viral genes, AC2 and AC3, which code for crucial viral protein functions. The idea was that the GM cassava would express the CRISPR-Cas9, which would knock out these two viral genes. Thus when the virus infected the cassava, it would be unable to spread in the plant.
But that’s not what happened. Mehta and Gruissem’s paper reveals that not only did the engineered CRISPR virus resistance completely fail but it also led to the emergence of a novel mutant virus that, if it had escaped, might have put at risk the entire cassava crop.
The authors concluded, “We have not yet tested the ability of the evolved virus to replicate independently. However, this mutant may also be an intermediate step towards the development of a truly pathogenic novel virus.”
The paper prompted a comment on Twitter by a representative of GARNet, a Cardiff-based “research network for the plant science community” funded by the UK’s public science funding body, the BBSRC. The GARNet person had a helpful suggestion: “Using multiple [RNA] guides should reduce chance of this happening.” Mehta responded, “Yep, or rather only delay it (geminiviruses recombine frequently), but for how long? And is that a risk we’re ok with in fields?”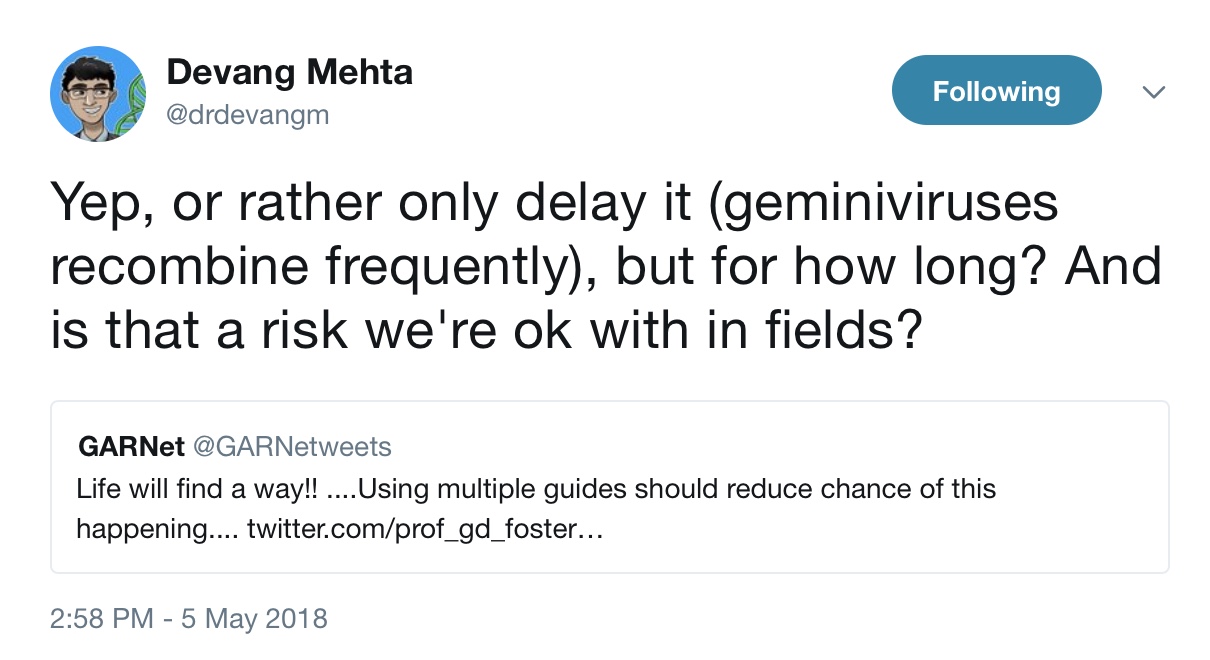
In his response, Mehta appears both disillusioned and cautious. He is right to be. He and his colleagues used CRISPR – a tool that is constantly hyped as precise and as giving rise only to predictable and intended changes – in this case to cure cassava viral infections. But they ended up making the virus potentially even more virulent.
Clearly their experiments have fully justified public concerns about crop genetic engineering technology. Mehta’s results illustrate yet again the unpredictability of the technology, even when using genome-editing tools that are claimed to only bring about precise and predictable changes to the plant’s DNA.
Nevertheless, one of the researchers – Mehta – had the temerity to publish his article attacking the public for what he claims are their baseless fears and suspicions. We could be forgiven for thinking that this represents a failure of logic – or perhaps even hypocrisy.
Non-GMO solutions work

In the long tale of self-delusion, hype, and risk that is the story of the (non-existent) GM virus-resistant cassava, one massive irony goes unremarked. According to experts, an important solution to cassava viruses is already out there, and it doesn’t involve GM.
Michael Farrelly, programme officer for the Tanzania-based Alliance for Food Sovereignty in Africa, said, “There is a lot of rubbish talked about CMD [cassava mosaic disease, caused by the CMV] and CBSD. A lot of the problem in Africa comes from diseased planting materials. The practical solution is not GM but improved access to quality planting material, coupled with better disease management practices.
“Cassava is not planted from a seed, but from cuttings, and it doesn’t store well, so commercial seed dealers tend not to sell it. It is generally traded less formally. The Chambezi agricultural station at Bagamoyo in Tanzania is doing a great job of setting up access to clean and disease-resistant planting material, initially from the station and then increasingly produced by local farmers under controlled conditions to ensure quality.”
The Food and Agriculture Organisation (FAO) agrees: “Strict quarantine procedures during international exchange of cassava germplasm and the use of resistant/tolerant varieties and virus-free planting material are key for the control of both CMD and CBSD in Africa.”
Regional Cassava Initiative
In 2009 the FAO launched the Regional Cassava Initiative, a four-year EU-funded project to develop and distribute new virus-resistant cassava varieties. These were non-GMO. At the end of the project in 2013 the FAO reported: “Agricultural research institutes initiated or completed their germplasm collection and set up multiplication plots of improved cassava planting materials. Disease-resistant and tolerant varieties of cassava were made widely available to vulnerable communities in the region. Farmers were sensitized and engaged through farmer groups, where they were trained on multiplication techniques, disease identification and nurseries maintenance.”[6]
The FAO report quotes Hemeri Mikidadi, a small-scale farmer in Hoyoyo, Tanzania, who participated in the project, as saying: “When our crops started to die, we were hungry. Our children had learning problems. We were trained on good agricultural practices. Now we leave enough space between our plants, we know when our cassava is affected by diseases and we take the right measures. My cassava is good and strong! We have enough to eat and our children are doing well at school.”[6]
The project met with challenges, notably the spread of CBSD, which has affected CMV-resistant varieties. An FAO video on the programme says, “While progress has been made, more effort is still needed.”
Non-GMO programmes starved of funding

Dr Angelika Hilbeck, a senior scientist at ETH Zurich who has many years’ experience of cassava research in Tanzania, explained: "Several generations of combined virus- and drought-resistant non-GMO cassava varieties have been available to farmers for many years in some regions of Tanzania. But the local breeders who work closely with farmers on these virus-resistant cassava varieties do so with so little support that they cannot possibly supply everybody and scale up the breeding and multiplication process to where it should be.
“That these virus-resistant cassava varieties exist and are handed out to farmers is well known, both in Tanzanian farming and research circles as well as in biotech circles. They get the little funding to multiply these varieties from the same donors that fund the GM cassava programme much more generously – the Gates Foundation and USAID – despite the GM cassava programme’s poor success rate and acceptability.
“This is not to say that viruses are not a problem; they definitely are. But farmers have been dealing with them for many years and together with breeders have developed working options to deal with them. What blocks these options from excelling is a lack of funding and institutional support, not a lack of potential. Instead the big money flows into a vision of an option that has not delivered anything tangible yet, while breeding has – even in the face of lack of funding.
"Technically the GM option will always be a risky one. We already know that the viruses evolve in a dynamic fashion. Any single-gene [virus resistance] option will likely be broken in a short time or of limited efficacy. But that is all that this technology can do to date, regardless of the genetic engineering technique used – new or old. This probably explains the poor success rate to date, despite the generous funding."
In this context, researchers’ and wealthy funders’ ongoing obsession with GM seems like a callous distraction from methods that are known to work but simply need more support to meet their goals.
Uncontrollable risks
In conclusion, it’s clear that the shrinkage of the GMO food venture in Europe is not due to the public’s anti-science attitudes but due to the inherent problems, limitations, and uncontrollable risks of GM technology. If genetic engineering produced even one genuinely useful crop, no amount of public “backlash and criticism” would hold it back from mass commercialisation. Mehta’s move to researching “more fundamental questions in plant biology” may prove more useful to society as well as more rewarding personally.
References
1. Hand E. Hungry African nations balk at biotech cassava: The politics of biotechnology. St Louis Post-Dispatch. http://nwrage.org/content/hungry-african-nations-balk-biotech-cassava. Published September 21, 2005.
2. Donald Danforth Plant Science Center. Danforth Center cassava viral resistance review update. June 2006. http://bit.ly/1ry2DUC.
3. Beyene G, Chauhan RD, Wagaba H, et al. Loss of CMD2-mediated resistance to cassava mosaic disease in plants regenerated through somatic embryogenesis. Mol Plant Pathol. 2016;17(7):1095-1110. doi:10.1111/mpp.12353
4. Food & Water Watch. Public Research, Private Gain: Corporate Influence over University Agricultural Research. Washington, DC: Food & Water Watch; 2012. http://www.foodandwaterwatch.org/news/public-research-private-gain-corporate-influence-over-university-agricultural-research.
5. Dunning R. A Synergistic Union or Selling out? University-Industry Relations, Biotechnology, and the UC-Berkeley/Novartis Partnership. Durham, NC: The Kenan Institute for Ethics at Duke University; 2009. https://bit.ly/2w8s2mu.
6. Food and Agriculture Organization (FAO). Managing Cassava Virus Diseases in Africa: The Regional Cassava Initiative. Rome, Italy: Food and Agriculture Organization (FAO); 2013. http://www.fao.org/fileadmin/user_upload/emergencies/docs/RCI%20Cassava%20brochure_ENG_FINAL.pdf.
Photos of cassavas within the body of this article are reproduced with kind permission of the late Wally Menne, Timberwatch.org. The photo of of the boy with cassava stems was taken in a village in the district of Kisarawe, Tanzania, south of Dar es Salaam. Kisarawe is a collection point for cassava roots brought in by local farmers. From there it is trucked to a processing plant in Dar es Salaam (see photo of truck).










